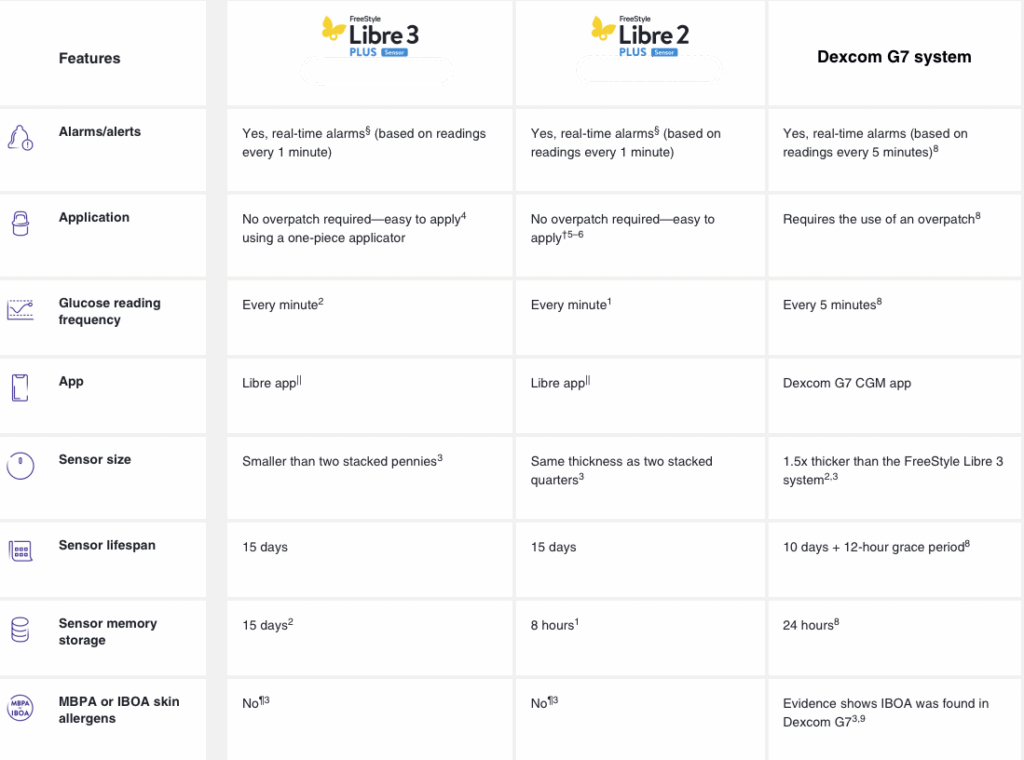
Abbott FreeStyle Libre CGM
Manage your diabetes with more confidence*1
EHCS carries all versions of the FreeStyle Libre system from Abbott Diabetes Care. The continuous glucose monitoring (CGM) system checks your blood sugar with a painless scan, instead of a routine fingerstick! No coding and no fingerstick calibration required, and customizable alerts and alarms are also available* with the FreeStyle Libre 2 Plus and FreeStyle Libre 3 Plus systems.
Newer sensors last for 15 days, require a 1-hour warm-up period, and work with the appropriate reader or compatible smartphone (click here to discover the universal Libre app).
The latest version, the FreeStyle Libre 3 Plus system, provides real-time glucose readings sent every minute to your smartphone† and can be viewed with a quick glance‡. For the FreeStyle Libre 3 Plus system, you will need to use a compatible smartphone or use the FreeStyle Libre 3 reader.

No fingerstick calibration or coding required

Customizable alarms and alerts available

Accurate sensor readings*

Backlit color touchscreen with reporting
———- Discover More ———-
Get to Know the System
Applying Your Sensor
Setting Up the Libre App
The FreeStyle Libre 3 Plus sensor is the latest innovation in the existing FreeStyle Libre 3 system. The FreeStyle Libre 3 Plus sensor is compatible* with the Libre app, the FreeStyle Libre 3 app, and FreeStyle Libre 3 reader. The complete system1 features the following:
-
Extends the sensor wear up to 15 days
-
Can work with insulin pumps
-
Expands the age indication to 2 years and older; is also indicated for pregnant women
Apply for a FreeStyle Libre 3 Plus system today!
* The FreeStyle Libre systems apps are only compatible with certain mobile devices and operating systems. Please verify with FreeStyle Libre support that your devices are compatible.
1 FreeStyle Libre 3 User’s Manual
The FreeStyle Libre 3 Plus sensor differs from the FreeStyle Libre 3 Plus sensor in the following ways:
- Can be worn up to 15 days
- Is indicated for children 2 years and older
- Works with Automated Insulin Delivery (AID) systems1
Whereas the FreeStyle Libre 3 sensor can be worn up to 14 days, is indicated for children 4 years and older, and does not work with AID systems.
The sensors look the same, so to identify if your sensor is a FreeStyle Libre 3 or FreeStyle Libre 3 Plus, you will need to use the Libre app or the FreeStyle Libre 3 app. A 14-day countdown bar means you have a FreeStyle Libre 3 sensor, and a 15-day countdown bar means you have a FreeStyle Libre 3 Plus sensor.
1 FreeStyle Libre 3 User’s Manual
* The FreeStyle Libre systems apps are only compatible with certain mobile devices and operating systems. Please visit the FreeStyle Libre Support site for more information about device compatibility before using the apps. Use of the FreeStyle Libre systems apps may require registration with LibreView.
No. Once your sensor is connected to your phone or reader, your glucose updates show up automatically in the app or when you get an alert.
The FDA requires a prescription for all versions of the CGM system. The FDA also requires a new prescription when you are moving from one to a different version (even if it’s from the FreeStyle Libre 2 to the FreeStyle Libre 3). EHCS will work with your healthcare team to obtain all required documentation!
Yes, you can. Click here to purchase online from EHCS. The FDA still requires a prescription even if you are paying out of pocket; EHCS will work with your healthcare team to obtain one on your behalf.
Yes, all FreeStyle Libre continuous glucose systems are FDA-approved CGM systems covered by Traditional Medicare (and Medicare Advantage plans) for those who qualify. Please read the Medicare criteria for more information. Most other commercial and government insurance plans have coverage criterial as well; reach out to your insurer for more information. We also welcome you to email our Diabetes Care team if you need assistance about CGM coverage.
*Fingersticks are required for treatment decisions: when you see Check Blood Glucose symbol, when symptoms do not match system readings, when you suspect readings may be inaccurate, or when you experience symptoms that may be due to high or low blood glucose.
*For indications and important safety information on all Abbott CGM systems, visit https://www.freestylelibre.us/safety-information.html
The FreeStyle Libre 14 day Flash Glucose Monitoring System is indicated for the management of diabetes in persons aged 18 and older.
FreeStyle Libre 2 and FreeStyle Libre 3 systems are indicated for use in people with diabetes ages 4 and older.
Medicare coverage is available for FreeStyle Libre systems if their respective readers are used to review glucose data on some days every month. Medicare and other third party payor criteria apply.
Abbott provides this information as a courtesy, it is subject to change and interpretation. The customer is ultimately responsible for determining the appropriate codes, coverage, and payment policies for individual patients. Abbott does not guarantee third party coverage or payment for our products or reimburse customers for claims that are denied by third party payors.
* Data from this study was collected with the outside US version of the FreeStyle Libre 14 day system. FreeStyle Libre 3 has the same features as FreeStyle Libre 14 day system with real-time glucose alarms. Therefore, the study data is applicable to both products.
† The FreeStyle Libre 3 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Use of the FreeStyle Libre 3 app requires registration with LibreView.
‡ 60-minute warm-up required when starting the sensor.
§ Among patient-applied sensors.
|| Notifications will only be received when alarms are turned on and the sensor is within 33 feet unobstructed of the reading device. You must enable the appropriate settings on your smartphone to receive alarms and alerts, see the FreeStyle Libre 3 User’s Manual for more information.
¶ The FreeStyle Libre 2 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Use of the FreeStyle Libre 2 app requires registration with LibreView.
# Notifications will only be received when alarms are turned on and the sensor is within 20 feet unobstructed of the reading device. You must enable the appropriate settings on your smartphone to receive alarms and alerts, see the FreeStyle Libre 2 User’s Manual for more information.
** The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose meter data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
†† The LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check https://www.librelinkup.com for more information about device compatibility before using the app. Use of the LibreLinkUp app requires registration with LibreView. LibreLinkUp is not intended to be used for dosing decisions. The user should follow instructions on the continuous glucose monitoring system. LibreLinkUp is not intended to replace self-monitoring practices as advised by a physician.
References:
1. Fokkert, Marion, et al. “Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4).” BMJ Open Diabetes Research and Care 7 (2019): e000809. https://doi.org/10.1136/bmjdrc-2019-000809.
2. FreeStyle Libre 3 User’s Manual.
3. Data on file. Comparison based on publicly available information.
4. Bolinder, Jan, et al. “Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial.” The Lancet 10057, no. 388 (September 2016): 2254-2263. https://doi.org/10.1016/S0140-6736(16)31535-5.
5. Yaron, Marianna, et al. “Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes.” Diabetes Care 42, no. 7 (July 2019); 1178-1184. https://doi.org/10.2337/dc18-0166.
6. Data on file, Abbott Diabetes Care.
7. FreeStyle Libre 2 User’s Manual.
Important Safety Information
FreeStyle Libre 2 and FreeStyle Libre 3 systems: Failure to use FreeStyle Libre 2 or FreeStyle Libre 3 systems as instructed in labeling may result in missing a severe low or high glucose event and/or making a treatment decision, resulting in injury. If glucose alarms and readings do not match symptoms or expectations, use a fingerstick value from a blood glucose meter for treatment decisions. Seek medical attention when appropriate or contact Abbott at 855-632-8658 or FreeStyleLibre.us for safety info.
The circular shape of the sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott. Other trademarks are the property of their respective owners.