Ready to upgrade your Medtronic pump?
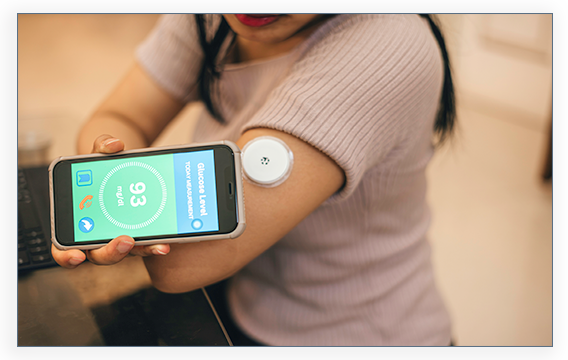
The MiniMed™ 770G™ system gives you the flexibility to continue living your life while managing your diabetes! Sometimes referred to as a hybrid closed loop system, it automatically adjusts delivery of basal (background) insulin based on CGM sensor glucose values. This means that the pump gets glucose readings from the CGM automatically, and then delivers a variable rate of insulin 24 hours a day based on your personal needs.** This integrated system – meaning having a pump and a CGM that speak to one another – may help reduce both high and low glucose levels.**
You can even stay up-to-date on your glucose trends and insulin delivery 24/7 using the MiniMed™ Mobile app for iOS and Android. This system adapts to your unique needs, so you can focus on living your life and worrying less about managing your diabetes!
Multiple Features Make Managing Your Diabetes Easier:
-
Data-sharing capability makes virtual and in-person doctor’s appointments easier
-
Smartguard™ Auto Mode helps prevent glucose highs and lows
-
MiniMed™ Mobile App allows you to discreetly check your numbers
-
Carelink™ Connect App brings more peace of mind for care partners
-
Exercise setting may reduce lows during activities
Now may be the best time to upgrade to the 770G system, and get access to Medtronic’s MiniMed™ 780G* software upgrade at no cost, when available. Software upgrades provide the latest pump features without having to switch your physical pump!
*Investigational. Not approved by the FDA for any use and not commercially available in the US. We will let you know as soon as the software upgrade is available with the option to add our future CGM!
**Refers to SmartGuard™ Auto Mode. Some user interaction required. Individual results may vary.
IMPORTANT SAFETY INFORMATION: MINIMED™ 770G SYSTEM WITH SMARTGUARD™ TECHNOLOGY
The MiniMed™ 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 770G System includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitoring (CGM) sensor glucose values (SG) and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 770G System consists of the following devices: MiniMed™ 770G Insulin Pump, the Guardian™ Link (3) Transmitter, the Guardian™ Sensor (3), one-press serter, the Accu-Chek® Guide Link blood glucose meter, and the Accu- Chek®Guide Test Strips. The system requires a prescription.
The Guardian™ Sensor (3) has not been evaluated and is not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ Sensor (3).
All therapy adjustments should be based on measurements obtained using the Accu-Chek® Guide Link blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the Accu-Chek® Guide Link blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
WARNING: Do not use the MiniMed™ 770G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 770G system.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 770G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult http://www.medtronicdiabetes.com/important-safety- information#minimed-770g and the appropriate user guide at http://www.medtronicdiabetes.com/download-library.